Search form
How to Live Better, Longer


This Trait Keeps You Going, Reduces Risk Of Death In Old Age: Study Reveals

Study Identifies A Factor That Affects Aging- But It's Out Of Your Control

Is Flexibility Key To Longevity? Study Links It To Survival In Middle-Aged Adults

Breakthrough Monthly Treatment May Boost Longevity, Vitality In Old Age: Mice Study Reveals

Exposure To Air Pollution During Pregnancy Quadruples Postpartum Depression Risk Up To 3 Years: Study

People Living With Brain Aneurysm At High Risk Of Anxiety, Depression: Study

Skipping Through Online Videos Can Boost Boredom, Try Watching Them Fully: Study

Friend's Genetic Traits Can Influence Your Mental Health Risk: Study

Explore The Healing Power Of Expressive Arts With Wellness Coach Karen Corona

Dr. Jason Shumard Revolutionizes Holistic Healing And Transformative Wellness

Thermal Earring To Monitor Temperature: Experts Say It Could Also Track Ovulation And Stress
First Saliva-Based Pregnancy Tests: Everything To Know

Children's Screen Time Mirrors Parents', Excessive Use Affects Language Skills: Study

Dry Eyes? Laughter May Be The Best Medicine, Says Study

Asthma Linked To Miscarriage And Fertility Issues In Women: Study

Excess Tummy Fat Linked To Widespread Chronic Pain, Particularly For Women: Study

SKNV is the First Pharmacy for Sensitive Skin

No Time to Exercise? How to Lose Weight and Get in the Best Shape You've Ever Been

How Red Borneo Kratom Boosts Your Productivity

Nolah Mattress Review: Is It a Good Buy?
How is oxygen used in the human body the complex journey, explained.

- Share on Twitter
- Share on Facebook
- Share on Pocket
Despite taking an average of 17,000 breaths each day, breathing is a process we all do without much thought or effort. However, the way your body processes the oxygen you need for survival is much more complex than you may imagine.
The TEDEd video below provides a detailed explanation of the journey oxygen endures as it travels through your entire body.
Read: Breaking Point: How Long Can Someone Go Without Breathing?
In order to survive, all of your cells need oxygen. The complx process of getting your body the oxygen it needs is a collaborative effort among your gut, brain, bones, lungs, blood, and heart.
As you unconsciously breathe in, the air around you enters through your nose and mouth, then begins on its roller coaster ride. The transportation route relies on red blood cells which contain oxygen-binding molecules called hemoglobin. Every second, your body churns about 2.5 million blood cells, so the oxygen sent to your lungs has a vast amount of transportation.
But, before getting to your lungs, your brain needs to initiate breathing by sending a message through your nervous system to your muscles, diaphragm, and ribs, which ultimately allows your lungs to expand.
Once your lungs process the oxygen, the oxygen-rich cells are carried to the cardiovascular network, which is a massive collection of blood vessels throughout your body. The network is so lengthy, that if stretched out, it would be able to wrap around the Earth several times. Lastly, thanks to your body's powerhouse known as your heart, the blood cells are propelled through your body to every single cell.
For a more comprehensive look at oxygen's journey, check out TEDEd’s video above.
See also: Oxygen Injected Into The Blood Via Microparticles May Soon Rescue Patients From Hypoxemia
Breathing In Extra Oxygen Shows Promise In Fighting Cancer And Boosting Immune System: But Will It Work In Humans?

- Alzheimer's
- Amputation/Prosthetics

- Dengue Fever
- Dental Health
- Dermatological Disorders
- Developmental Disorders
- Digestive Disorders
- Down Syndrome

- Gastrointestinal Disorders
- Genetic Disorders
- Genital Warts
- Geriatric care
- Gerontology
- Gum Disease
- Gynecological Disorders
- Head And Neck Cancer

- Kidney Cancer
- Kidney Disease
- Knee Problems
- Lead Poisoning
- Liver Disease
- Low Testosterone
- Lung Cancer

- Macular Degeneration
- Men's Health
- Menstruation/Periods
- Mental Health
- Metabolic Disorders

- Pancreatic Cancer
- Parasitic Infections
- Parkinson's Disease
- Pediatric Diseases

- Schizophrenia
- Senior Health
- Sexual Health
- Sickle Cell Disease
- Skin Cancer
- Sleep Apnea

- Uterine Cancer
- Varicose Veins
- Viral Infection
- Women's Health
- Yeast Infection
Want a daily email of lesson plans that span all subjects and age groups?
Oxygen’s surprisingly complex journey through your body - enda butler.
3,099,893 Views
39,935 Questions Answered
Let’s Begin…
Oxygen forms about 21% of the air around us. In your body, oxygen forms a vital role in the production of energy in most cells. But if gases can only efficiently diffuse across tiny distances, how does oxygen reach the cells deep inside your body? Enda Butler tracks the surprisingly complex journey of oxygen through your body.
About TED-Ed Animations
TED-Ed Animations feature the words and ideas of educators brought to life by professional animators. Are you an educator or animator interested in creating a TED-Ed Animation? Nominate yourself here »
Meet The Creators
- Educator Enda Butler
- Script Editor Emma Bryce
- Director Dalibor Rajninger
- Producer Vessela Dantcheva
- Animator Dalibor Rajninger
- Designer Dalibor Rajninger
- Illustrator Dalibor Rajninger
- Composer Alexander Daniel, Alexander Evtimov, Mihail Yosifov
- Sound Designer Alexander Daniel, Alexander Evtimov, Mihail Yosifov
- Associate Producer Jessica Ruby
- Content Producer Gerta Xhelo
- Editorial Producer Alex Rosenthal
- Narrator Addison Anderson
More from Getting Under Our Skin

What does appendix pain feel like?
Lesson duration 05:38
425,688 Views

Why is it so dangerous to step on a rusty nail?
Lesson duration 04:47
2,908,470 Views

What are warts — and how do you get rid of them?
Lesson duration 05:14
348,918 Views

Why does hitting your funny bone feel so horrible?
Lesson duration 04:19
480,379 Views

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
66 Oxygen transport
Learning Objectives
After reading this chapter you should be able to-
- Describe the ways in which oxygen is transported in blood and explain the relative importance of each to total oxygen transport.
- State the reversible chemical equation for oxygen binding to hemoglobin and predict how raising or lowering the partial pressure of oxygen will shift the equilibrium.
- Interpret the oxygen-hemoglobin saturation curve at low and high partial pressures of oxygen.
- Explain the changes in hemoglobin affinity for oxygen when the curve shifts to the right or the left.
- List factors that shift the oxygen-hemoglobin saturation curve to the right and explain how this results in increased oxygen release at the tissues.
- List factors that shift the oxygen-hemoglobin saturation curve to the left and explain how this facilitates oxygen binding to hemoglobin in the lungs.
The other major activity in the lungs is the process of respiration , the process of gas exchange. The function of respiration is to provide oxygen for use by body cells during cellular respiration and to eliminate carbon dioxide, a waste product of cellular respiration, from the body. In order for the exchange of oxygen and carbon dioxide to occur, both gases must be transported between the external and internal respiration sites. Although carbon dioxide is more soluble than oxygen in blood, both gases require a specialized transport system for the majority of the gas molecules to be moved between the lungs and other tissues.
Oxygen Transport in the Blood
Even though oxygen is transported via the blood, you may recall that oxygen is not very soluble in liquids. A small amount of oxygen does dissolve in the blood and is transported in the bloodstream, but it is only about 1.5% of the total amount. The majority of oxygen molecules are carried from the lungs to the body’s tissues by a specialized transport system, which relies on the erythrocyte—the red blood cell. Erythrocytes contain hemoglobin , which serves to bind oxygen molecules to the erythrocyte ( Figure 66.1 ). Heme is the portion of hemoglobin that contains iron, and it is heme that binds to oxygen. One hemoglobin molecule contains four iron-containing Heme molecules, and because of this, each hemoglobin molecule is capable of carrying up to four molecules of oxygen. As oxygen diffuses across the respiratory membrane from the alveolus to the capillary, it also diffuses into the red blood cell and is bound by hemoglobin. The following reversible chemical reaction describes the production of the final product, oxyhemoglobin (Hb–O 2 ), which is formed when oxygen binds to hemoglobin. Oxyhemoglobin is a bright red-colored molecule that contributes to the bright red color of oxygenated blood.
In this formula, Hb represents reduced hemoglobin, that is, hemoglobin that does not have oxygen bound to it. There are multiple factors involved in how readily heme binds to and dissociates from oxygen, which will be discussed in the subsequent sections.
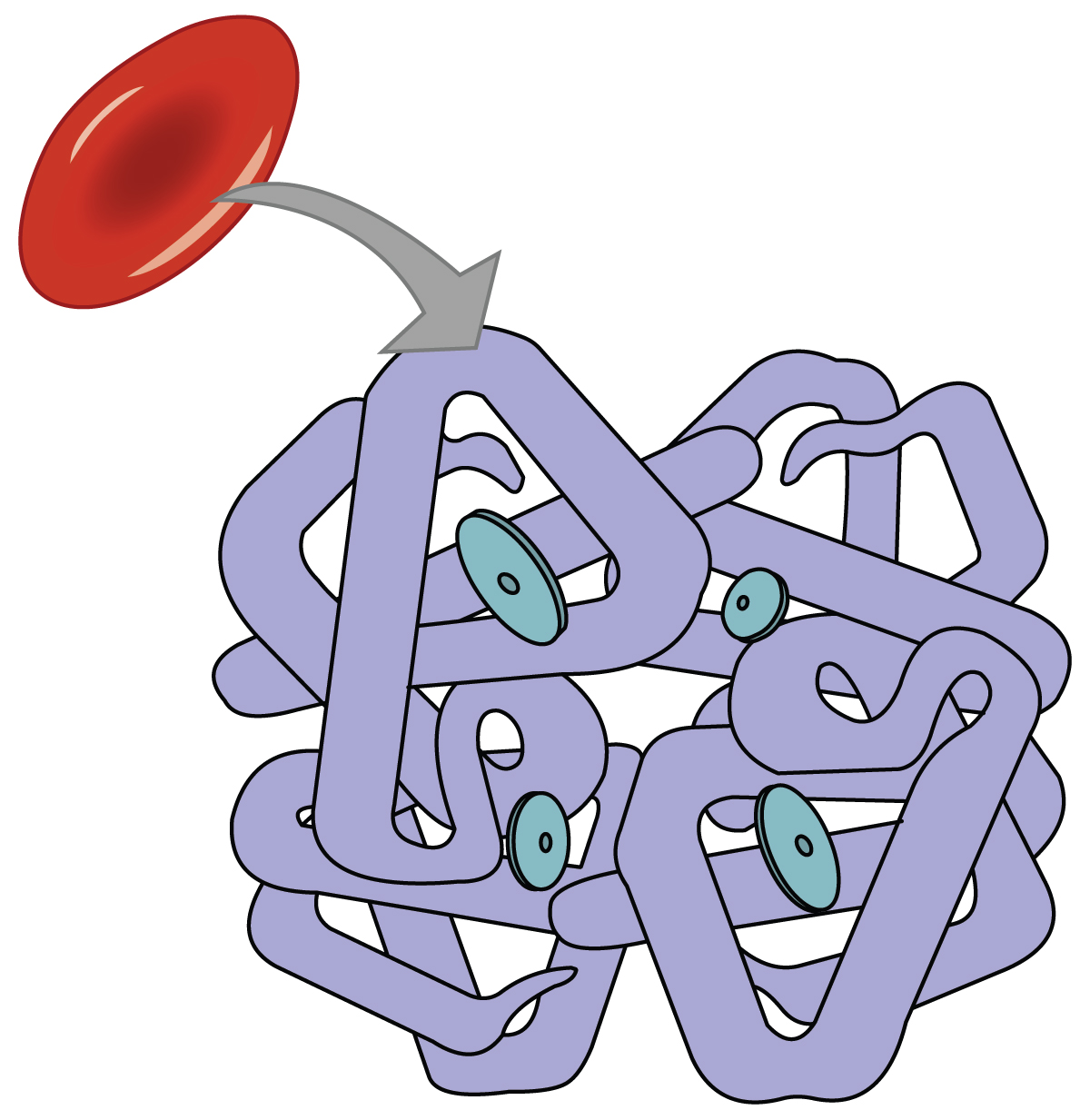
Function of Hemoglobin
Hemoglobin is composed of subunits, a protein structure that is referred to as a quaternary structure. Each of the four subunits that make up hemoglobin is arranged in a ring-like fashion, with an iron atom covalently bound to the heme in the center of each subunit. Binding of the first oxygen molecule causes a conformational change in hemoglobin that allows the second molecule of oxygen to bind more readily. As each molecule of oxygen is bound, it further facilitates the binding of the next molecule, until all four heme sites are occupied by oxygen. The opposite occurs as well: After the first oxygen molecule dissociates and is “dropped off” at the tissues, the next oxygen molecule dissociates more readily. When all four heme sites are occupied, the hemoglobin is said to be saturated. When one to three heme sites are occupied, the hemoglobin is said to be partially saturated. Therefore, when considering the blood as a whole, the percent of the available heme units that are bound to oxygen at a given time is called hemoglobin saturation . Hemoglobin saturation of 100 percent means that every heme unit in all of the erythrocytes of the body is bound to oxygen. In a healthy individual with normal hemoglobin levels, hemoglobin saturation generally ranges from 95 percent to 99 percent.
Oxygen Dissociation from Hemoglobin
Partial pressure is an important aspect of the binding of oxygen to and disassociating from heme. An oxygen–hemoglobin dissociation curve is a graph that describes the relationship of partial pressure to the binding of oxygen to heme and its subsequent dissociation from heme ( Figure 66.2 ). Remember that gases travel from an area of higher partial pressure to an area of lower partial pressure. In addition, the affinity of an oxygen molecule for heme increases as more oxygen molecules are bound. Therefore, in the oxygen–hemoglobin saturation curve, as the partial pressure of oxygen increases, a proportionately greater number of oxygen molecules are bound by heme. Not surprisingly, the oxygen–hemoglobin saturation/dissociation curve also shows that the lower the partial pressure of oxygen, the fewer oxygen molecules are bound to heme. As a result, the partial pressure of oxygen plays a major role in determining the degree of binding of oxygen to heme at the site of the respiratory membrane, as well as the degree of dissociation of oxygen from heme at the site of body tissues.
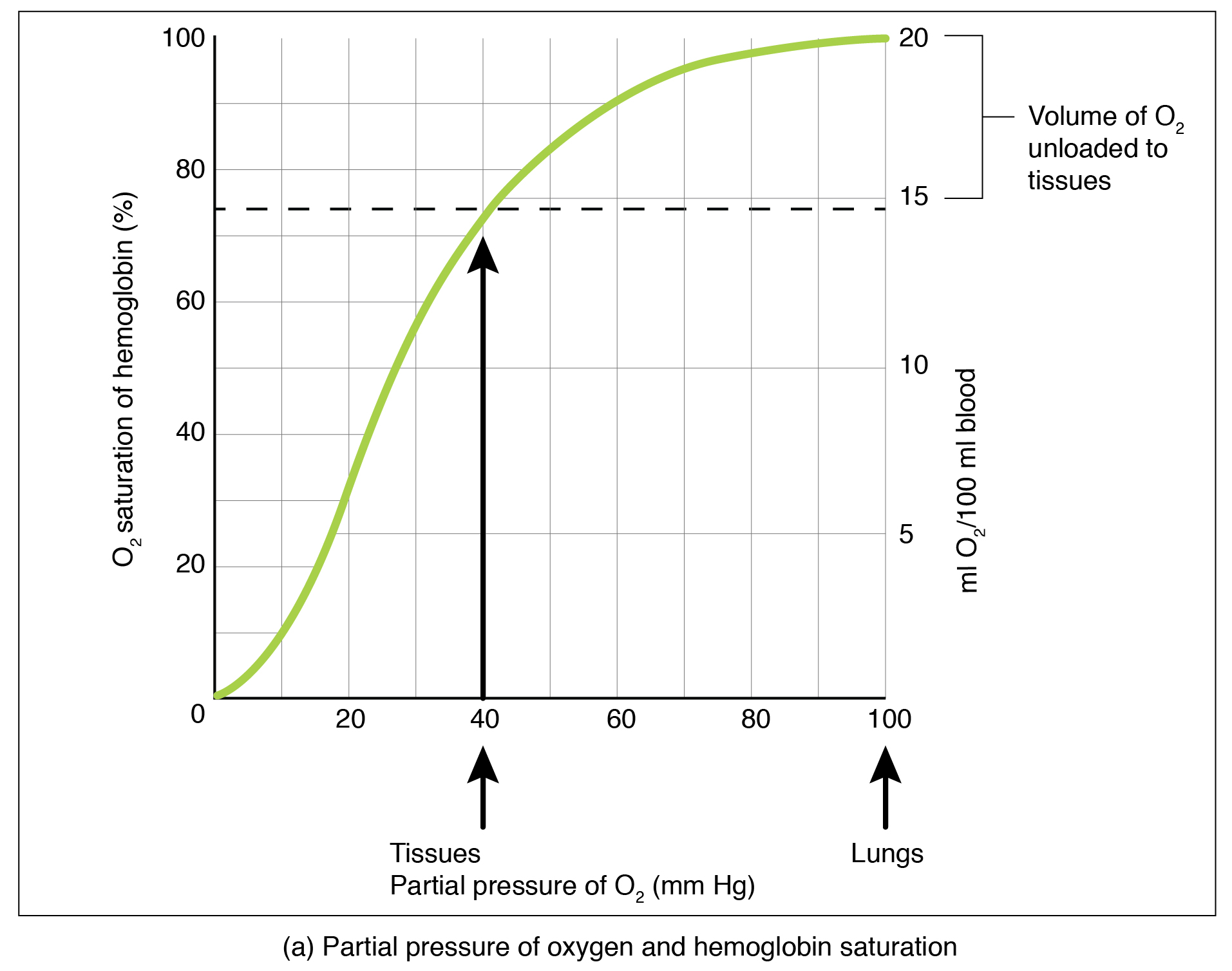
The mechanisms behind the oxygen–hemoglobin saturation/dissociation curve also serve as automatic control mechanisms that regulate how much oxygen is delivered to different tissues throughout the body. This is important because some tissues have a higher metabolic rate than others. Highly active tissues, such as muscle, rapidly use oxygen to produce ATP, lowering the partial pressure of oxygen in the tissue to about 20 mm Hg. The partial pressure of oxygen inside capillaries is about 100 mm Hg, so the difference between the two becomes quite high, about 80 mm Hg. As a result, a greater number of oxygen molecules dissociate from hemoglobin and enter the tissues. The reverse is true of tissues, such as adipose (body fat), which have lower metabolic rates. Because less oxygen is used by these cells, the partial pressure of oxygen within such tissues remains relatively high, resulting in fewer oxygen molecules dissociating from hemoglobin and entering the tissue interstitial fluid. Although venous blood is said to be deoxygenated, some oxygen is still bound to hemoglobin in its red blood cells. This provides an oxygen reserve that can be used when tissues suddenly demand more oxygen.
Factors Affecting the Oxygen–Hemoglobin Saturation/Dissociation Curve
Factors other than partial pressure also affect the oxygen–hemoglobin saturation/dissociation curve. For example, a higher temperature promotes hemoglobin and oxygen to dissociate faster, whereas a lower temperature inhibits dissociation ( Figure 66.2b ). However, the human body tightly regulates temperature, so this factor may not affect gas exchange throughout the body. The exception to this is in highly active tissues, which may release a larger amount of energy than is given off as heat. As a result, oxygen readily dissociates from hemoglobin, which is a mechanism that helps to provide active tissues with more oxygen.
The pH of the blood is another factor that influences the oxygen–hemoglobin saturation/dissociation curve ( Figure 66.2 ). The Bohr effect is a phenomenon that arises from the relationship between pH and oxygen’s affinity for hemoglobin: A lower, more acidic pH promotes oxygen dissociation from hemoglobin. In contrast, a higher, or more basic, pH inhibits oxygen dissociation from hemoglobin. The greater the amount of carbon dioxide in the blood, the more molecules that must be converted, which in turn generates hydrogen ions and thus lowers blood pH. Furthermore, blood pH may become more acidic when certain byproducts of cell metabolism, such as lactic acid, carbonic acid, and carbon dioxide, are released into the bloodstream.
the process of gas exchange
iron-containing protein in red blood cells that facilitates oxygen transportation
the form of hemoglobin carrying oxygen
a measure of available heme units bound to oxygen (%)
a graphical representation of the relationship between the partial pressure of oxygen in arterial blood and the percent saturation of oxygen to heme
the phenomenon describing the relationship between pH and oxyhemoglobin saturation; hemoglobin and oxygen have lower affinities in more acidic environments
Basic Human Physiology Copyright © by Jim Davis is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Oxygen transport—1....
Oxygen transport—1. Basic principles
- Related content
- Peer review
- D F Treacher ,
Mammalian life and the bioenergetic processes that maintain cellular integrity depend on a continuous supply of oxygen to sustain aerobic metabolism. Reduced oxygen delivery and failure of cellular use of oxygen occur in various circumstances and if not recognised result in organ dysfunction and death. Prevention, early identification, and correction of tissue hypoxia are essential skills. An understanding of the key steps in oxygen transport within the body is essential to avoid tissue hypoxia.
Red blood cells entering a capillary
- Download figure
- Open in new tab
- Download powerpoint
Physiology of oxygen transport
Although oxygen is the substrate that cells use in the greatest quantity and on which aerobic metabolism and cell integrity depend, the tissues have no storage system for oxygen. They rely on a continuous supply at a rate that precisely matches changing metabolic requirements. If this supply fails, even for a few minutes, tissue hypoxaemia may develop resulting in anaerobic metabolism and production of lactate.
Key steps in oxygen cascade
Uptake in the lungs
Carrying capacity of blood
Global delivery from lungs to tissue
Regional distribution of oxygen delivery
Diffusion from capillary to cell
Cellular use of oxygen
Oxygen transport from atmosphere to mitochondria. Values given in parenthesis are for a healthy 70 kg adult breathing air (FiO 2 0.21) at standard barometric pressure
Partial pressures of oxygen at sea level (101 kPa)
- View inline
Oxygen transport from environmental air to the mitochondria of individual cells occurs as a series of steps. The heart, lungs, and circulation extract oxygen from the atmosphere and generate a flow of oxygenated blood to the tissues to maintain aerobic metabolism. The system must be energy efficient (avoiding unnecessary cardiorespiratory work), match oxygen distribution with metabolic demand, and allow efficient oxygen transport across the extravascular tissue matrix. At the tissue level, cells must extract oxygen from the extracellular environment and use it efficiently in cellular metabolic processes.
Oxygen uptake in the lungs
Arterial oxygen tension (PaO 2 ) is determined by inspired oxygen concentration and barometric pressure, alveolar ventilation, diffusion of oxygen from alveoli to pulmonary capillaries, and distribution and matching of ventilation and perfusion.
Inspired oxygen concentration and barometric pressure
The percentage of oxygen in atmospheric air is constant at 21% and does not change with altitude. Atmospheric pressure is the sum of the partial pressures of the constituent gases, oxygen and nitrogen, and varies with the weather and altitude.
Effect of tidal volume on ventilation
If minute volume is 6 l/min, respiratory rate is 10 breaths/min, tidal volume 0.6 l, and dead space 0.15 l:
V=10×0.6−0.15=4.5 l, which is adequate
If minute volume is 6 l/min, respiratory rate 30 breaths/min, tidal volume 0.2 l, and dead space 0.15 l:
VA=30×0.2−0.15=1.5 l, which is inadequate
Alveolar ventilation
Ventilation of the alveoli is essential if alveolar oxygen pressure (PAO 2 ) is to be maintained and carbon dioxide removed. Alveolar ventilation (VA) depends on the rate of breathing and the tidal volume (TV). A normal tidal volume of 600 ml results in alveolar ventilation of 450 ml, with 150 ml to overcome the physiological dead space of the tracheobronchial tree. At very low tidal volumes the dead space alone may be ventilated even though the minute volume (rate x tidal volume) is normal due to a high respiratory rate. Alveolar hypoventilation is reflected by a fall in alveolar and arterial PO 2 with increasing PaCO 2 .
Aims of treating alveolar hypoventilation in chronic obstructive pulmonary disease
Correct the cause of respiratory pump failure
Improve efficiency of ventilation
Reduce the work of breathing through bronchodilators or use of invasive or non-invasive ventilation
Diffusion from alveoli to pulmonary capillaries
The PAO 2 provides the driving pressure for diffusion into the pulmonary capillary blood and in normal conditions is the main determinant of the partial pressure of oxygen in arterial blood (PaO 2 ). The PAO 2 -PaO 2 (A-a) gradient describes the overall efficiency of oxygen uptake from alveolar gas to arterial blood in the lungs. It is normally less than 1 kPa but may exceed 60 kPa in severe respiratory failure.
Effect of true shunt (Qs/Qt) and effective shunt (ventilation-perfusion mismatch) on relation between arterial and inspired oxygen partial pressures
The capillary blood is usually fully oxygenated before it has traversed one third of the distance of the alveolar-capillary interface. Inadequate oxygenation because of a shortened pulmonary capillary transit time occurs only with very high cardiac output or severe desaturation of mixed pulmonary arterial blood. Impaired diffusion of oxygen from alveolar gas to pulmonary capillary blood is uncommon. Arterial hypoxaemia in fibrotic lung disease is related more to ventilation-perfusion mismatching than to thickening of the alveolar wall.
Causes of arterial hypoxaemia
Alveolar hypoventilation
Respiratory depression from sedation or analgesia
Respiratory muscle weakness:
Prolonged mechanical ventilation
Catabolic effects of critical illness
Muscle relaxants or steroids
Phrenic nerve damage (cardiac surgery or trauma)
Neuromuscular disorders (Guillain-Barré, etc)
Obstructive airways disease
Pulmonary oedema
Acute respiratory distress syndrome (particularly with fibrosis in later stages)
Ventilation-perfusion mismatch
Alveolar collapse
Acute respiratory distress syndrome
Pneumothorax
Drugs—pulmonary vasodilators
Distribution and matching of ventilation and perfusion
Efficient gas exchange requires matching of alveolar ventilation and perfusion. Inadequate ventilation of perfused alveoli or reduced perfusion of well ventilated alveoli impairs reoxygenation of pulmonary arterial blood and is termed ventilation-perfusion (V/Q) mismatch.
The net effect of abnormalities in the distribution of ventilation and perfusion is calculated as the venous admixture (Qs/Qt), which includes “true” shunt (mixed venous blood that completely bypasses the pulmonary capillary bed) and “effective” shunt due to ventilation-perfusion mismatch. Venous admixture is normally less than 5% of the cardiac output and is reflected by a low A-a gradient. A “true shunt” above 30% of total pulmonary blood flow will greatly lower PaO 2 . In these circumstances increasing inspired PO 2 will have little effect on PaO 2 . Similar reductions in PaO 2 due to ventilation-perfusion mismatch respond to oxygen.
Management of arterial hypoxaemia
Appropriate management of arterial hypoxaemia due to failure of oxygen uptake in the lungs depends on the underlying cause.
Supplemental oxygen
The mechanism responsible for hypoxaemia (PaO 2 <8 kPa) in critically ill patients may not be obvious. Immediate supplemental oxygen is essential. Small increases in PaO 2 may produce valuable increases in oxygen saturation and delivery to tissues because of the shape of the oxygen dissociation curve. The increasing hypercapnia and respiratory acidosis seen in critically ill patients is not caused by oxygen treatment but by progression of the underlying respiratory problem and the patient's inability to sustain the work of breathing. Reduction of oxygen, in the mistaken belief that ventilatory drive will increase and PaCO 2 fall, will increase hypoxaemia and risks cardiorespiratory arrest. The only exception to this is certain patients with chronic obstructive pulmonary disease who require controlled oxygen to avoid carbon dioxide retention.
Incentive spirometer
Physiotherapy
Alveolar collapse and hypoventilation may increase ventilation-perfusion mismatch and can be corrected by mobilisation, increased clearance of secretions, enhancing tidal breaths by sitting the patient up to improve diaphragmatic descent, and the use of ventilatory aids such as the incentive spirometer.

Non-invasive ventilation
Non-invasive techniques have been a considerable advance in the treatment of patients with respiratory pump failure or alveolar collapse. These techniques may prevent the need for invasive mechanical ventilation and allow earlier extubation of the ventilated patient.
Benefits and disadvantages of continuous positive airways pressure
Recruitment of collapsed alveoli
Increase in end-expiratory lung volume
Reduced A-a gradient
Improved lung compliance
Reduced work of breathing
Disadvantages
High FiO 2 can conceal severity of condition
Discourages coughing and clearance of secretions
Risk of aspiration
Continuous positive airways pressure is valuable for patients with low lung volumes (alveolar collapse, pulmonary oedema, pneumonia) but should be avoided in patients with bronchospasm and at risk of gas trapping. The simplest system involves a flow generator connected to a wall oxygen supply that entrains air to achieve a FiO 2 of 0.3-1.0. The gas flow is connected to the patient via a face or nasal mask and to a valve that opens with a predetermined pressure in the range 2.5-10 cm H 2 O. Provided the gas flow exceeds the patient's maximum inspiratory flow rate, the exit valve is held open and the selected pressure is obtained throughout the circuit and airway.
Patient receiving oxygen by continuous positive airways pressure
Non-invasive positive pressure ventilation is delivered by using a portable ventilator with inbuilt compressor that entrains room air to generate pressures greater than 20 cm H 2 O during inspiration. This increases tidal and minute volumes and reduces the patient's respiratory workload. The technique is suitable for patients with respiratory pump failure and chronic obstructive pulmonary disease. A nasal mask is often used, but a chin strap may be required to prevent excessive flow through the mouth. The patient requires reassurance and careful matching of the timing and pressure of ventilation to their respiratory pattern.
Biphasic positive airways pressure combines the benefits of the two techniques above. It delivers two levels of pressure in phase with respiration. The higher pressure provides the inspiratory pressure support and the lower pressure is maintained during expiration, increasing functional residual capacity. It is indicated for patients who require both assistance with the work of breathing and improved ventilation-perfusion matching.
Mechanical ventilation of critically ill patient in intensive care
Mechanical ventilation
In over 60% of patients appropriately admitted to intensive care units non-invasive strategies will not achieve adequate PaO 2 and formal mechanical ventilation is needed. By this stage impaired gas exchange will often be caused by both ventilation-perfusion mismatch and alveolar hypoventilation.
Mechanical ventilation eliminates the metabolic cost of breathing, which is normally less than 5% of the total oxygen consumption (VO 2 ) but may rise to 30% in critically ill patients. It allows the patient to be sedated, given analgesia, and if necessary paralysed, which further reduces VO 2 .
The timing, pressure, and flow characteristics of the respiratory cycle may be controlled to recruit alveoli, minimise the ventilation-perfusion mismatch, and improve arterial oxygenation. The FiO 2 should be less than 0.8 to avoid the collapse of low ventilation-perfusion lung units and to reduce the risk of oxygen toxicity and pulmonary fibrosis.
Addition of nitric oxide to inspired gas in a mechanical ventilator. Inspired concentration of nitric oxide and expired concentration of nitrogen dioxide must be measured
Specialised techniques to improve arterial oxygenation
When conventional strategies fail to achieve acceptable arterial oxygenation other recently introduced techniques may be tried:
Nitric oxide added to the inspired gas in low concentrations (1-20 parts per million) vasodilates the pulmonary vascular bed adjacent to ventilated alveoli, which improves ventilation-perfusion matching. It is rapidly scavenged by haemoglobin and therefore does not produce systemic vasodilation. Although arterial oxygenation may improve greatly, the response is unpredictable and rebound hypoxaemia may occur on withdrawal. The benefit is not clinically proved, and the potential risk of lung toxicity is not established.
Prone position— Hypoxaemia may occur in supine ventilated patients who develop severe dependent consolidation with a large shunt fraction. Turning the patient from supine to prone improves gas exchange in about half of patients through redistribution of ventilation. It can also improve drainage of secretions from previously dependent areas of lung. Response usually occurs within 15 minutes.
Severe dependent consolidation in patient with acute respiratory distress syndrome which may improve with prone positioning
Extracorporeal membrane oxygenation is a final option in patients with unacceptable arterial hypoxaemia despite the use of other techniques. Venous blood is passed through a membrane oxygenator at up to 4 l/min and returned 100% saturated with oxygen and with 50% of carbon dioxide removed. This can provide 50% of the total oxygen requirement and allows FiO 2 , airways pressure, and tidal volume to be reduced, resting the lungs and reducing the risk of ventilator induced lung injury. Its benefit is proved for neonates but its value in adults is unclear.
Oxygen carrying capacity of the blood
Most oxygen is carried in the blood attached to haemoglobin with only a small amount (typically less than 2% if PaO 2 <14 kPa) dissolved in the plasma. Despite this, the optimum haemoglobin concentration in critically ill patients is 100-110 g/l, which represents the balance between maximising oxygen content and the adverse microcirculatory effects associated with the marked rise in viscosity that occurs at higher packed cell volumes.
Effect of increasing levels of supplemental oxygen and transfusion in an anaemic hypoxaemic patient showing importance of saturation and haemoglobin concentration
Oxygen delivery from lungs to tissue
The major function of the central circulation is to transport oxygen from the lungs to the peripheral tissues at a rate that satisfies overall oxygen consumption. Failure of this component of the oxygen cascade to supply sufficient oxygen to meet the metabolic requirements of the tissues defines circulatory shock. Under normal resting conditions the total or “global” oxygen delivery (DO 2 ) is more than adequate to meet the total tissue oxygen requirements (VO 2 ) for aerobic metabolism. DO 2 is defined as the product of cardiac output (Qt) and oxygen content of blood (CaO 2 ). CaO 2 is derived from the saturation (SaO 2 ), haemoglobin content (Hb), and a constant K (the coefficient for haemoglobin-oxygen binding capacity). Thus DO 2 (ml/min)=Qt×Hb×SaO 2 ×K.
Mechanisms causing failure of global oxygen transport
Reduction in cardiac output (for example, heart failure) results in “low flow” tissue hypoxia
Fall in haemoglobin concentration or failure of haemoglobin mediated oxygen binding or release (for example, haemoglobinopathy) produces “anaemic” tissue hypoxia
Failure of oxygen uptake by blood (for example, inadequate ventilation, ventilation-perfusion mismatching, low FiO 2 ) results in “hypoxic” tissue hypoxia
The importance of oxygen delivery in the management of critically ill patients depends on its relation with oxygen consumption. The sum of the oxygen consumptions by the various organs is the global oxygen consumption (VO 2 ), which can be measured directly or derived from measures of cardiac output (Qt) and arterial and venous oxygen contents: VO 2 =Qt×(CaO 2 −CvO 2 ).
The amount of oxygen consumed (VO 2 ) as a fraction of oxygen delivery (DO 2 ) defines the oxygen extraction ratio (VO 2 /DO 2 ).
In a normal 70 kg adult undertaking normal daily activity VO 2 would be 250 ml/min with an oxygen extraction ratio of 25%. The oxygen not extracted by the tissues returns to the lungs and the mixed venous saturation (SvO 2 ) measured in the pulmonary artery represents the pooled venous saturation from all organs. SvO 2 will be influenced by changes in both DO 2 and VO 2 , but provided that regional perfusion and the mechanisms for cellular oxygen uptake are normal it will be >65% if the supply matches demand.
Oxygen saturation in blood draining from different organs varies widely (hepatic venous saturation is 30-40% and renal venous saturation about 80%) and reflects both oxygen delivery and metabolic demands of these tissues
As metabolic demand (VO 2 ) increases or supply (DO 2 ) diminishes, the oxygen extraction ratio rises to maintain aerobic metabolism. However, once the maximum extraction ratio is reached (at 60-70% for most tissues) further increases in demand or falls in supply lead to hypoxia. In critically ill patients, however, the slope of maximum oxygen extraction ratio is less steep, reflecting the reduced extraction of oxygen by tissues, and does not plateau so that consumption remains supply dependent even at “supranormal” levels of oxygen delivery.
Relation between oxygen delivery (DO 2 ) and oxygen consumption (VO 2 ) in normal subject (solid line) and critically ill patient (dotted line)
A large study of postoperative patients reported that increasing global oxygen delivery above normal levels increased oxygen consumption and improved survival. This demonstration of “supply dependence” led to the hypothesis that critically ill patients had covert oxygen debt that could be eliminated by increasing oxygen supply and spawned the era of “goal directed therapy.” Cardiac output was increased to achieve oxygen supply rates above 600 ml/min/m 2 by aggressive volume loading and the use of vasodilating inotropes.
Although increasing the oxygen supply by volume replacement in relatively volume depleted postoperative patients is appropriate, recent European studies have shown that it may be detrimental in adequately resuscitated patients presenting with incipient or established multiorgan failure. Aggressive fluid loading may impair pulmonary gas exchange and reduce oxygen diffusion in tissues due to increased endothelial permeability and myocardial dysfunction in inflammatory and septic conditions in critically ill patients. The increase in mortality associated with the use of pulmonary artery catheters may reflect the adverse effects of their use in attempting to achieve supranormal levels of oxygen delivery.
Acknowledgments
D F Treacher is consultant physician in intensive care, Guy's and St Thomas's Hospital Trust, London
The ABC of Oxygen is edited by Richard M Leach, consultant physician, department of intensive care, and P John Rees, consultant physician, department of respiratory medicine, Guy's and St Thomas's Hospitals Trust, London.
The picture of red blood cells was obtained from Science Photo Library.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases & Disorders Education Program
- Publications and Resources
- Clinical Trials
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Ask a Scientist
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- Health Topics
- < Back To How the Lungs Work
- The Respiratory System
- How Your Body Controls Breathing
- What Breathing Does for the Body
- How to Keep Your Lungs Healthy
MORE INFORMATION
How the Lungs Work The Respiratory System
Language switcher.
Your lungs are on each side of your heart, inside your chest cavity. They are the main organs of the respiratory system. The right lung is divided into three lobes (sections), and the left lung is divided into two lobes. Your left lung is slightly smaller than your right lung, since your heart takes up some space on the left side. When you breathe in, air enters your airways and travels down into the air sacs, or alveoli, in your lungs. This is where gas exchange takes place.
An animation walks through the structures of the lungs. Medical Animation Copyright © 2022 Nucleus Medical Media Inc. All rights reserved.
The circulatory system, which is made up of the heart and blood vessels, supports the respiratory system by bringing blood to and from the lungs. The circulatory system helps deliver nutrients and oxygen from the lungs to tissues and organs throughout the body. It also helps remove carbon dioxide and waste products. Other body systems that work with the respiratory system include the nervous system, lymphatic system , and immune system.

The airways are pipes that carry oxygen-rich air to the alveoli in your lungs. They also carry the waste gas carbon dioxide out of your lungs. The airways include these body parts:
- Mouth
- Nose and linked air passages called the nasal cavity and sinuses
- Larynx (voice box)
- Trachea (windpipe)
- Tubes called bronchial tubes , or bronchi, and their branches
- Smaller tubes called bronchioles that branch off of the bronchial tubes
Learn what happens in your lungs when you breathe in and breathe out. Medical Animation Copyright © 2022 Nucleus Medical Media Inc. All rights reserved.
Air comes into your body
Air first enters your body through your nose or mouth, which moistens and warms the air since cold, dry air can irritate your lungs. The air then travels past your voice box and down your windpipe. Rings of tough tissue, called cartilage, acts as a support to keep the bronchial tubes open.
Inside your lungs, the bronchial tubes branch into thousands of thinner tubes called bronchioles. The bronchioles end in clusters of tiny air sacs called alveoli.
Air fills your lung’s air sacs
Your lungs have about 150 million alveoli. Normally, your alveoli are elastic, meaning that their size and shape can change easily. Alveoli are able to easily expand and contract because their insides are coated with a substance called surfactant. Surfactant reduces the work it takes to breathe by helping the lungs inflate more easily when you breathe in. It also prevents the lungs from collapsing when you breathe out.
Each of these alveoli is made up of a mesh of tiny blood vessels called capillaries. The capillaries connect to a network of arteries and veins that move blood through your body.
Blood low in oxygen flows through the lungs
The pulmonary artery and its branches deliver blood to the capillaries that surround the alveoli. This blood is rich in carbon dioxide and low in oxygen.
Oxygen flows into your blood
Carbon dioxide moves from the blood into the air inside the alveoli. At the same time, oxygen moves from the air into the blood in the capillaries.

The lungs are surrounded by the pleura, a membrane with two layers. The space between these two layers is called the pleural cavity. A slippery liquid called pleural fluid acts as a lubricant to reduce friction during breathing.
22.5 Transport of Gases
Learning objectives.
By the end of this section, you will be able to:
- Describe the principles of oxygen transport
- Describe the structure of hemoglobin
- Compare and contrast fetal and adult hemoglobin
- Describe the principles of carbon dioxide transport
The other major activity in the lungs is the process of respiration, the process of gas exchange. The function of respiration is to provide oxygen for use by body cells during cellular respiration and to eliminate carbon dioxide, a waste product of cellular respiration, from the body. In order for the exchange of oxygen and carbon dioxide to occur, both gases must be transported between the external and internal respiration sites. Although carbon dioxide is more soluble than oxygen in blood, both gases require a specialized transport system for the majority of the gas molecules to be moved between the lungs and other tissues.
Oxygen Transport in the Blood
Even though oxygen is transported via the blood, you may recall that oxygen is not very soluble in liquids. A small amount of oxygen does dissolve in the blood and is transported in the bloodstream, but it is only about 1.5% of the total amount. The majority of oxygen molecules are carried from the lungs to the body’s tissues by a specialized transport system, which relies on the erythrocyte—the red blood cell. Erythrocytes contain a metalloprotein, hemoglobin, which serves to bind oxygen molecules to the erythrocyte ( Figure 22.25 ). Heme is the portion of hemoglobin that contains iron, and it is heme that binds oxygen. Each hemoglobin molecule contains four iron-containing heme molecules, and because of this, one hemoglobin molecule is capable of carrying up to four molecules of oxygen. As oxygen diffuses across the respiratory membrane from the alveolus to the capillary, it also diffuses into the red blood cell and is bound by hemoglobin. The following reversible chemical reaction describes the production of the final product, oxyhemoglobin (HbO 2 ), which is formed when oxygen binds to hemoglobin. Oxyhemoglobin is a bright red-colored molecule that contributes to the bright red color of oxygenated blood.
In this formula, Hb represents reduced hemoglobin, that is, hemoglobin that does not have oxygen bound to it. There are multiple factors involved in how readily heme binds to and dissociates from oxygen, which will be discussed in the subsequent sections.
Function of Hemoglobin
Hemoglobin is composed of subunits, a protein structure that is referred to as a quaternary structure. Each of the four subunits that make up hemoglobin is arranged in a ring-like fashion, with an iron atom covalently bound to the heme in the center of each subunit. Binding of the first oxygen molecule causes a conformational change in hemoglobin that allows the second molecule of oxygen to bind more readily. As each molecule of oxygen is bound, it further facilitates the binding of the next molecule, until all four heme sites are occupied by oxygen. The opposite occurs as well: After the first oxygen molecule dissociates and is “dropped off” at the tissues, the next oxygen molecule dissociates more readily. When all four heme sites are occupied, the hemoglobin is said to be saturated. When one to three heme sites are occupied, the hemoglobin is said to be partially saturated. Therefore, when considering the blood as a whole, the percent of the available heme units that are bound to oxygen at a given time is called hemoglobin saturation. Hemoglobin saturation of 100 percent means that every heme unit in all of the erythrocytes of the body is bound to oxygen. In a healthy individual with normal hemoglobin levels, hemoglobin saturation generally ranges from 95 percent to 99 percent.
Oxygen Dissociation from Hemoglobin
Partial pressure is an important aspect of the binding of oxygen to and disassociation from heme. An oxygen–hemoglobin dissociation curve is a graph that describes the relationship of partial pressure to the binding of oxygen to heme and its subsequent dissociation from heme ( Figure 22.26 ). Remember that gases travel from an area of higher partial pressure to an area of lower partial pressure. In addition, the affinity of an oxygen molecule for heme increases as more oxygen molecules are bound. Therefore, in the oxygen–hemoglobin saturation curve, as the partial pressure of oxygen increases, a proportionately greater number of oxygen molecules are bound by heme. Not surprisingly, the oxygen–hemoglobin saturation/dissociation curve also shows that the lower the partial pressure of oxygen, the fewer oxygen molecules are bound to heme. As a result, the partial pressure of oxygen plays a major role in determining the degree of binding of oxygen to heme at the site of the respiratory membrane, as well as the degree of dissociation of oxygen from heme at the site of body tissues.
The mechanisms behind the oxygen–hemoglobin saturation/dissociation curve also serve as automatic control mechanisms that regulate how much oxygen is delivered to different tissues throughout the body. This is important because some tissues have a higher metabolic rate than others. Highly active tissues, such as muscle, rapidly use oxygen to produce ATP, lowering the partial pressure of oxygen in the tissue to about 20 mm Hg. The partial pressure of oxygen inside capillaries is about 100 mm Hg, so the difference between the two becomes quite high, about 80 mm Hg. As a result, a greater number of oxygen molecules dissociate from hemoglobin and enter the tissues. The reverse is true of tissues, such as adipose (body fat), which have lower metabolic rates. Because less oxygen is used by these cells, the partial pressure of oxygen within such tissues remains relatively high, resulting in fewer oxygen molecules dissociating from hemoglobin and entering the tissue interstitial fluid. Although venous blood is said to be deoxygenated, some oxygen is still bound to hemoglobin in its red blood cells. This provides an oxygen reserve that can be used when tissues suddenly demand more oxygen.
Factors other than partial pressure also affect the oxygen–hemoglobin saturation/dissociation curve. For example, a higher temperature promotes hemoglobin and oxygen to dissociate faster, whereas a lower temperature inhibits dissociation (see Figure 22.26 , middle ). However, the human body tightly regulates temperature, so this factor may not affect gas exchange throughout the body. The exception to this is in highly active tissues, which may release a larger amount of energy than is given off as heat. As a result, oxygen readily dissociates from hemoglobin, which is a mechanism that helps to provide active tissues with more oxygen.
Certain hormones, such as androgens, epinephrine, thyroid hormones, and growth hormone, can affect the oxygen–hemoglobin saturation/disassociation curve by stimulating the production of a compound called 2,3-bisphosphoglycerate (BPG) by erythrocytes. BPG is a byproduct of glycolysis. Because erythrocytes do not contain mitochondria, glycolysis is the sole method by which these cells produce ATP. BPG promotes the disassociation of oxygen from hemoglobin. Therefore, the greater the concentration of BPG, the more readily oxygen dissociates from hemoglobin, despite its partial pressure.
The pH of the blood is another factor that influences the oxygen–hemoglobin saturation/dissociation curve (see Figure 22.26 ). The Bohr effect is a phenomenon that arises from the relationship between pH and oxygen’s affinity for hemoglobin: A lower, more acidic pH promotes oxygen dissociation from hemoglobin. In contrast, a higher, or more basic, pH inhibits oxygen dissociation from hemoglobin. The greater the amount of carbon dioxide in the blood, the more molecules that must be converted, which in turn generates hydrogen ions and thus lowers blood pH. Furthermore, blood pH may become more acidic when certain byproducts of cell metabolism, such as lactic acid, carbonic acid, and carbon dioxide, are released into the bloodstream.
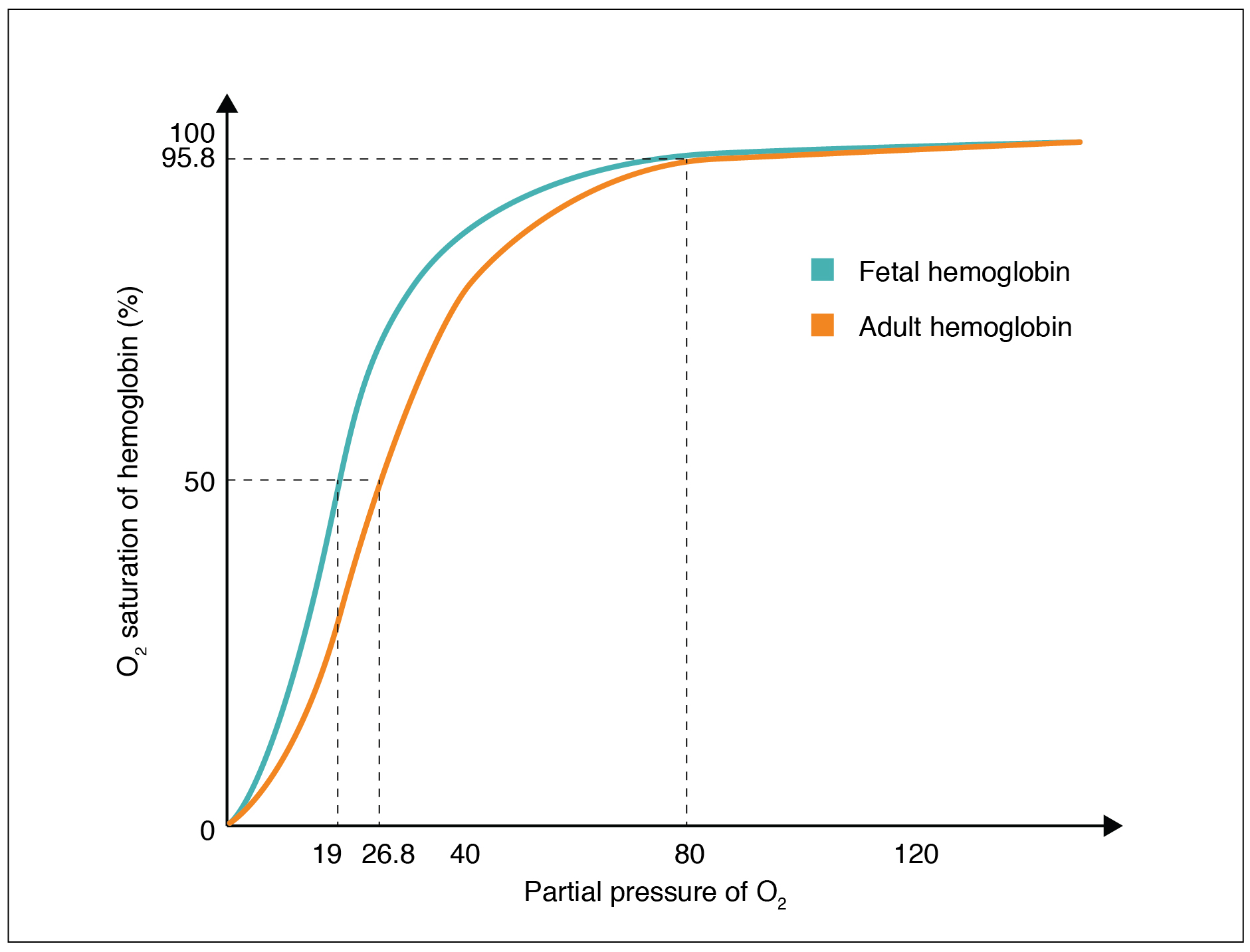
Hemoglobin of the Fetus
The fetus has its own circulation with its own erythrocytes; however, it is dependent on the pregnant person for oxygen. Blood is supplied to the fetus by way of the umbilical cord, which is connected to the placenta and separated from maternal blood by the chorion. The mechanism of gas exchange at the chorion is similar to gas exchange at the respiratory membrane. However, the partial pressure of oxygen is lower in the maternal blood in the placenta, at about 35 to 50 mm Hg, than it is in maternal arterial blood. The difference in partial pressures between maternal and fetal blood is not large, as the partial pressure of oxygen in fetal blood at the placenta is about 20 mm Hg. Therefore, there is not as much diffusion of oxygen into the fetal blood supply. The fetus’ hemoglobin overcomes this problem by having a greater affinity for oxygen than maternal hemoglobin ( Figure 22.27 ). Both fetal and adult hemoglobin have four subunits, but two of the subunits of fetal hemoglobin have a different structure that causes fetal hemoglobin to have a greater affinity for oxygen than does adult hemoglobin.
Carbon Dioxide Transport in the Blood
Carbon dioxide is transported by three major mechanisms. The first mechanism of carbon dioxide transport is by blood plasma, as some carbon dioxide molecules dissolve in the blood. The second mechanism is transport in the form of bicarbonate (HCO 3 – ), which also dissolves in plasma. The third mechanism of carbon dioxide transport is similar to the transport of oxygen by erythrocytes ( Figure 22.28 ).
Dissolved Carbon Dioxide
Although carbon dioxide is not considered to be highly soluble in blood, a small fraction—about 7 to 10 percent—of the carbon dioxide that diffuses into the blood from the tissues dissolves in plasma. The dissolved carbon dioxide then travels in the bloodstream and when the blood reaches the pulmonary capillaries, the dissolved carbon dioxide diffuses across the respiratory membrane into the alveoli, where it is then exhaled during pulmonary ventilation.
Bicarbonate Buffer
A large fraction—about 70 percent—of the carbon dioxide molecules that diffuse into the blood is transported to the lungs as bicarbonate. Most bicarbonate is produced in erythrocytes after carbon dioxide diffuses into the capillaries, and subsequently into red blood cells. Carbonic anhydrase (CA) causes carbon dioxide and water to form carbonic acid (H 2 CO 3 ), which dissociates into two ions: bicarbonate (HCO 3 – ) and hydrogen (H + ). The following formula depicts this reaction:
Bicarbonate tends to build up in the erythrocytes, so that there is a greater concentration of bicarbonate in the erythrocytes than in the surrounding blood plasma. As a result, some of the bicarbonate will leave the erythrocytes and move down its concentration gradient into the plasma in exchange for chloride (Cl – ) ions. This phenomenon is referred to as the chloride shift and occurs because by exchanging one negative ion for another negative ion, neither the electrical charge of the erythrocytes nor that of the blood is altered.
At the pulmonary capillaries, the chemical reaction that produced bicarbonate (shown above) is reversed, and carbon dioxide and water are the products. Much of the bicarbonate in the plasma re-enters the erythrocytes in exchange for chloride ions. Hydrogen ions and bicarbonate ions join to form carbonic acid, which is converted into carbon dioxide and water by carbonic anhydrase. Carbon dioxide diffuses out of the erythrocytes and into the plasma, where it can further diffuse across the respiratory membrane into the alveoli to be exhaled during pulmonary ventilation.
Carbaminohemoglobin
About 20 percent of carbon dioxide is bound by hemoglobin and is transported to the lungs. Carbon dioxide does not bind to iron as oxygen does; instead, carbon dioxide binds amino acid moieties on the globin portions of hemoglobin to form carbaminohemoglobin , which forms when hemoglobin and carbon dioxide bind. When hemoglobin is not transporting oxygen, it tends to have a bluish-purple tone to it, creating the darker maroon color typical of deoxygenated blood. The following formula depicts this reversible reaction:
Similar to the transport of oxygen by heme, the binding and dissociation of carbon dioxide to and from hemoglobin is dependent on the partial pressure of carbon dioxide. Because carbon dioxide is released from the lungs, blood that leaves the lungs and reaches body tissues has a lower partial pressure of carbon dioxide than is found in the tissues. As a result, carbon dioxide leaves the tissues because of its higher partial pressure, enters the blood, and then moves into red blood cells, binding to hemoglobin. In contrast, in the pulmonary capillaries, the partial pressure of carbon dioxide is high compared to within the alveoli. As a result, carbon dioxide dissociates readily from hemoglobin and diffuses across the respiratory membrane into the air.
In addition to the partial pressure of carbon dioxide, the oxygen saturation of hemoglobin and the partial pressure of oxygen in the blood also influence the affinity of hemoglobin for carbon dioxide. The Haldane effect is a phenomenon that arises from the relationship between the partial pressure of oxygen and the affinity of hemoglobin for carbon dioxide. Hemoglobin that is saturated with oxygen does not readily bind carbon dioxide. However, when oxygen is not bound to heme and the partial pressure of oxygen is low, hemoglobin readily binds to carbon dioxide.
Interactive Link
Watch this video to see the transport of oxygen from the lungs to the tissues. Why is oxygenated blood bright red, whereas deoxygenated blood tends to be more of a purple color?
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/anatomy-and-physiology-2e/pages/1-introduction
- Authors: J. Gordon Betts, Kelly A. Young, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble, Peter DeSaix
- Publisher/website: OpenStax
- Book title: Anatomy and Physiology 2e
- Publication date: Apr 20, 2022
- Location: Houston, Texas
- Book URL: https://openstax.org/books/anatomy-and-physiology-2e/pages/1-introduction
- Section URL: https://openstax.org/books/anatomy-and-physiology-2e/pages/22-5-transport-of-gases
© Jun 13, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Overview of the Respiratory System
To sustain life, the body must produce sufficient energy. Energy is produced by burning molecules in food, which is done by the process of oxidation (whereby food molecules are combined with oxygen). Oxidation involves carbon and hydrogen being combined with oxygen to form carbon dioxide and water. The consumption of oxygen and the production of carbon dioxide are thus indispensable to life. It follows that the human body must have an organ system designed to eliminate carbon dioxide from the circulating blood and absorb oxygen from the atmosphere at a rate rapid enough for the body’s needs, even during peak exercise. The respiratory system enables oxygen to enter the body and carbon dioxide to leave the body.
The respiratory system starts at the nose and mouth and continues through the airways and the lungs. Air enters the respiratory system through the nose and mouth and passes down the throat (pharynx) and through the voice box, or larynx. The entrance to the larynx is covered by a small flap of tissue, the epiglottis, that automatically closes during swallowing, thus preventing food or drink from entering the airways.
The trachea (windpipe) is the largest airway. The trachea branches into two smaller airways: the left and right mainstem [or main] bronchi.
Each lung is divided into sections (lobes): three in the right lung and two in the left lung. The left lung is a little smaller than the right lung because it shares space in the left side of the chest with the heart.
Inside the Lungs and Airways
The bronchi themselves branch many times into smaller airways, ending in the narrowest airways (bronchioles), which are as small as one half of a millimeter (or 2/100 of an inch) across. The airways resemble an upside-down tree, which is why this part of the respiratory system is often called the bronchial tree. Large airways are held open by semiflexible, fibrous connective tissue called cartilage. Smaller airways are supported by the lung tissue that surrounds and is attached to them. The walls of the smaller airways have a thin, circular layer of smooth muscle. The airway muscle can relax or contract, thus changing airway size.
Thousands of alveoli (small air sacs) are at the end of each bronchiole. Together, the millions of alveoli of the lungs form a surface of more than 100 square meters (1111 square feet). Within the alveolar walls is a dense network of tiny blood vessels called capillaries. The extremely thin barrier between air and capillaries allows oxygen to move from the alveoli into the blood and allows carbon dioxide to move from the blood in the capillaries into the air in the alveoli.
The pleura is a slippery membrane that covers the lungs as well as the inside of the chest wall. It allows the lungs to move smoothly during breathing and as the person moves. Normally, the two layers of the pleura have only a small amount of lubricating fluid between them. The two layers glide smoothly over each other as the lungs change size and shape.
Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Cookie Preferences

22.5 Transport of Gases
Learning objectives.
By the end of this section, you will be able to:
- Describe the principles of oxygen transport
- Describe the structure of hemoglobin
- Compare and contrast fetal and adult hemoglobin
- Describe the principles of carbon dioxide transport
The other major activity in the lungs is the process of respiration, the process of gas exchange. The function of respiration is to provide oxygen for use by body cells during cellular respiration and to eliminate carbon dioxide, a waste product of cellular respiration, from the body. In order for the exchange of oxygen and carbon dioxide to occur, both gases must be transported between the external and internal respiration sites. Although carbon dioxide is more soluble than oxygen in blood, both gases require a specialized transport system for the majority of the gas molecules to be moved between the lungs and other tissues.
Oxygen Transport in the Blood
Even though oxygen is transported via the blood, you may recall that oxygen is not very soluble in liquids. A small amount of oxygen does dissolve in the blood and is transported in the bloodstream, but it is only about 1.5% of the total amount. The majority of oxygen molecules are carried from the lungs to the body’s tissues by a specialized transport system, which relies on the erythrocyte—the red blood cell. Erythrocytes contain a metalloprotein, hemoglobin, which serves to bind oxygen molecules to the erythrocyte ( Figure 22.5.1 ). Heme is the portion of hemoglobin that contains iron, and it is heme that binds oxygen. One hemoglobin molecule contains iron-containing Heme molecules, and because of this, each hemoglobin molecule is capable of carrying up to four molecules of oxygen. As oxygen diffuses across the respiratory membrane from the alveolus to the capillary, it also diffuses into the red blood cell and is bound by hemoglobin. The following reversible chemical reaction describes the production of the final product, oxyhemoglobin (Hb–O 2 ), which is formed when oxygen binds to hemoglobin. Oxyhemoglobin is a bright red-colored molecule that contributes to the bright red color of oxygenated blood.
In this formula, Hb represents reduced hemoglobin, that is, hemoglobin that does not have oxygen bound to it. There are multiple factors involved in how readily heme binds to and dissociates from oxygen, which will be discussed in the subsequent sections.

Function of Hemoglobin
Hemoglobin is composed of subunits, a protein structure that is referred to as a quaternary structure. Each of the four subunits that make up hemoglobin is arranged in a ring-like fashion, with an iron atom covalently bound to the heme in the center of each subunit. Binding of the first oxygen molecule causes a conformational change in hemoglobin that allows the second molecule of oxygen to bind more readily. As each molecule of oxygen is bound, it further facilitates the binding of the next molecule, until all four heme sites are occupied by oxygen. The opposite occurs as well: After the first oxygen molecule dissociates and is “dropped off” at the tissues, the next oxygen molecule dissociates more readily. When all four heme sites are occupied, the hemoglobin is said to be saturated. When one to three heme sites are occupied, the hemoglobin is said to be partially saturated. Therefore, when considering the blood as a whole, the percent of the available heme units that are bound to oxygen at a given time is called hemoglobin saturation. Hemoglobin saturation of 100 percent means that every heme unit in all of the erythrocytes of the body is bound to oxygen. In a healthy individual with normal hemoglobin levels, hemoglobin saturation generally ranges from 95 percent to 99 percent.
Oxygen Dissociation from Hemoglobin
Partial pressure is an important aspect of the binding of oxygen to and disassociation from heme. An oxygen–hemoglobin dissociation curve is a graph that describes the relationship of partial pressure to the binding of oxygen to heme and its subsequent dissociation from heme ( Figure 22.5.2 ). Remember that gases travel from an area of higher partial pressure to an area of lower partial pressure. In addition, the affinity of an oxygen molecule for heme increases as more oxygen molecules are bound. Therefore, in the oxygen–hemoglobin saturation curve, as the partial pressure of oxygen increases, a proportionately greater number of oxygen molecules are bound by heme. Not surprisingly, the oxygen–hemoglobin saturation/dissociation curve also shows that the lower the partial pressure of oxygen, the fewer oxygen molecules are bound to heme. As a result, the partial pressure of oxygen plays a major role in determining the degree of binding of oxygen to heme at the site of the respiratory membrane, as well as the degree of dissociation of oxygen from heme at the site of body tissues.

The mechanisms behind the oxygen–hemoglobin saturation/dissociation curve also serve as automatic control mechanisms that regulate how much oxygen is delivered to different tissues throughout the body. This is important because some tissues have a higher metabolic rate than others. Highly active tissues, such as muscle, rapidly use oxygen to produce ATP, lowering the partial pressure of oxygen in the tissue to about 20 mm Hg. The partial pressure of oxygen inside capillaries is about 100 mm Hg, so the difference between the two becomes quite high, about 80 mm Hg. As a result, a greater number of oxygen molecules dissociate from hemoglobin and enter the tissues. The reverse is true of tissues, such as adipose (body fat), which have lower metabolic rates. Because less oxygen is used by these cells, the partial pressure of oxygen within such tissues remains relatively high, resulting in fewer oxygen molecules dissociating from hemoglobin and entering the tissue interstitial fluid. Although venous blood is said to be deoxygenated, some oxygen is still bound to hemoglobin in its red blood cells. This provides an oxygen reserve that can be used when tissues suddenly demand more oxygen.
Factors other than partial pressure also affect the oxygen–hemoglobin saturation/dissociation curve. For example, a higher temperature promotes hemoglobin and oxygen to dissociate faster, whereas a lower temperature inhibits dissociation (see Figure 22.5.2 , middle ). However, the human body tightly regulates temperature, so this factor may not affect gas exchange throughout the body. The exception to this is in highly active tissues, which may release a larger amount of energy than is given off as heat. As a result, oxygen readily dissociates from hemoglobin, which is a mechanism that helps to provide active tissues with more oxygen.
Certain hormones, such as androgens, epinephrine, thyroid hormones, and growth hormone, can affect the oxygen–hemoglobin saturation/disassociation curve by stimulating the production of a compound called 2,3-bisphosphoglycerate (BPG) by erythrocytes. BPG is a byproduct of glycolysis. Because erythrocytes do not contain mitochondria, glycolysis is the sole method by which these cells produce ATP. BPG promotes the disassociation of oxygen from hemoglobin. Therefore, the greater the concentration of BPG, the more readily oxygen dissociates from hemoglobin, despite its partial pressure.
The pH of the blood is another factor that influences the oxygen–hemoglobin saturation/dissociation curve (see Figure 22.5.2 ). The Bohr effect is a phenomenon that arises from the relationship between pH and oxygen’s affinity for hemoglobin: A lower, more acidic pH promotes oxygen dissociation from hemoglobin. In contrast, a higher, or more basic, pH inhibits oxygen dissociation from hemoglobin. The greater the amount of carbon dioxide in the blood, the more molecules that must be converted, which in turn generates hydrogen ions and thus lowers blood pH. Furthermore, blood pH may become more acidic when certain byproducts of cell metabolism, such as lactic acid, carbonic acid, and carbon dioxide, are released into the bloodstream.
Hemoglobin of the Fetus
The fetus has its own circulation with its own erythrocytes; however, it is dependent on the mother for oxygen. Blood is supplied to the fetus by way of the umbilical cord, which is connected to the placenta and separated from maternal blood by the chorion. The mechanism of gas exchange at the chorion is similar to gas exchange at the respiratory membrane. However, the partial pressure of oxygen is lower in the maternal blood in the placenta, at about 35 to 50 mm Hg, than it is in maternal arterial blood. The difference in partial pressures between maternal and fetal blood is not large, as the partial pressure of oxygen in fetal blood at the placenta is about 20 mm Hg. Therefore, there is not as much diffusion of oxygen into the fetal blood supply. The fetus’ hemoglobin overcomes this problem by having a greater affinity for oxygen than maternal hemoglobin ( Figure 22.5.3 ). Both fetal and adult hemoglobin have four subunits, but two of the subunits of fetal hemoglobin have a different structure that causes fetal hemoglobin to have a greater affinity for oxygen than does adult hemoglobin.
Carbon Dioxide Transport in the Blood
Carbon dioxide is transported by three major mechanisms. The first mechanism of carbon dioxide transport is by blood plasma, as some carbon dioxide molecules dissolve in the blood. The second mechanism is transport in the form of bicarbonate (HCO 3 – ), which also dissolves in plasma. The third mechanism of carbon dioxide transport is similar to the transport of oxygen by erythrocytes ( Figure 22.5.4 ).
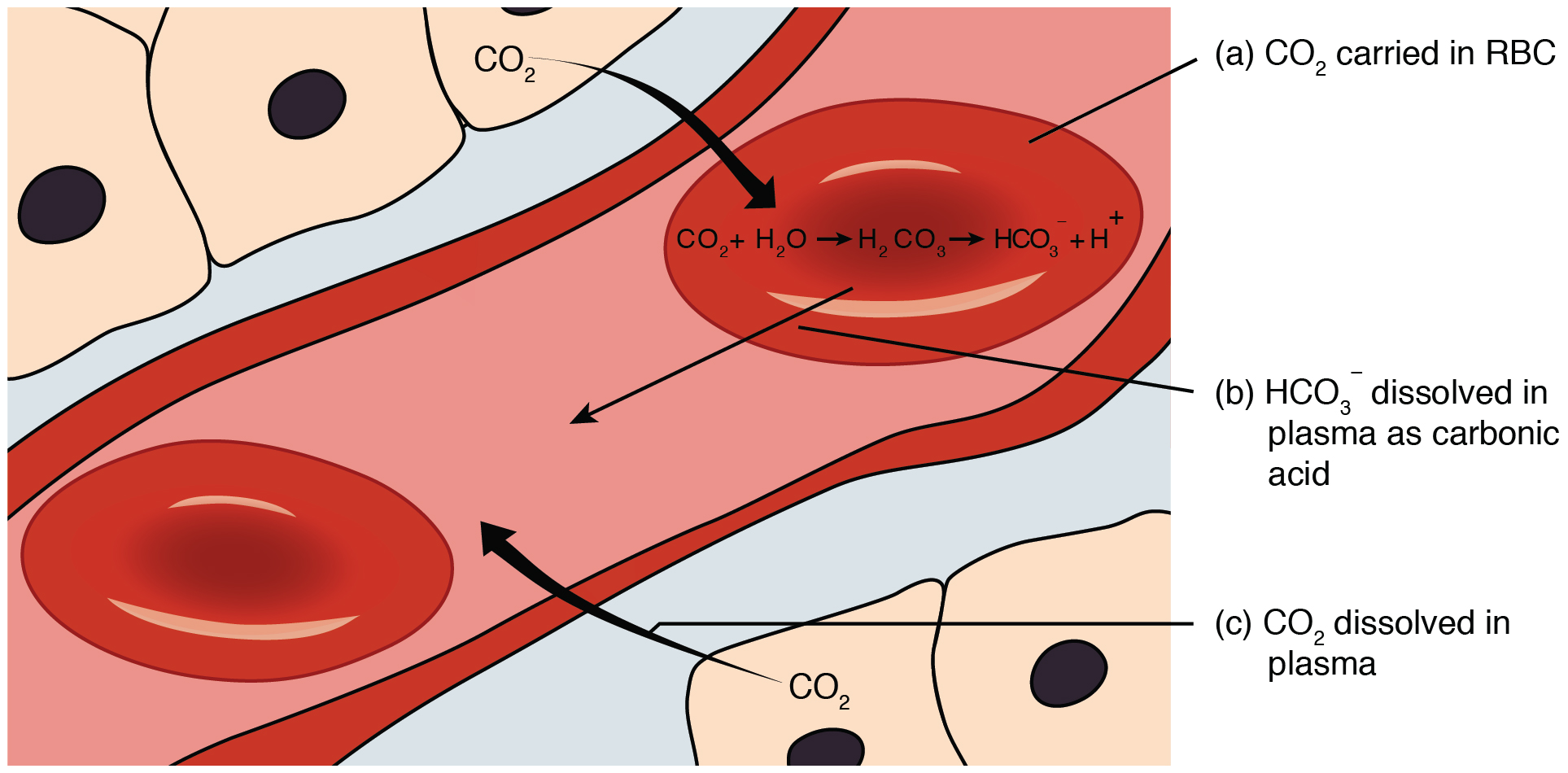
Dissolved Carbon Dioxide
Although carbon dioxide is not considered to be highly soluble in blood, a small fraction—about 7 to 10 percent—of the carbon dioxide that diffuses into the blood from the tissues dissolves in plasma. The dissolved carbon dioxide then travels in the bloodstream and when the blood reaches the pulmonary capillaries, the dissolved carbon dioxide diffuses across the respiratory membrane into the alveoli, where it is then exhaled during pulmonary ventilation.
Bicarbonate Buffer
A large fraction—about 70 percent—of the carbon dioxide molecules that diffuse into the blood is transported to the lungs as bicarbonate. Most bicarbonate is produced in erythrocytes after carbon dioxide diffuses into the capillaries, and subsequently into red blood cells. Carbonic anhydrase (CA) causes carbon dioxide and water to form carbonic acid (H 2 CO 3 ), which dissociates into two ions: bicarbonate (HCO 3 – ) and hydrogen (H + ). The following formula depicts this reaction:
Bicarbonate tends to build up in the erythrocytes, so that there is a greater concentration of bicarbonate in the erythrocytes than in the surrounding blood plasma. As a result, some of the bicarbonate will leave the erythrocytes and move down its concentration gradient into the plasma in exchange for chloride (Cl – ) ions. This phenomenon is referred to as the chloride shift and occurs because by exchanging one negative ion for another negative ion, neither the electrical charge of the erythrocytes nor that of the blood is altered.
At the pulmonary capillaries, the chemical reaction that produced bicarbonate (shown above) is reversed, and carbon dioxide and water are the products. Much of the bicarbonate in the plasma re-enters the erythrocytes in exchange for chloride ions. Hydrogen ions and bicarbonate ions join to form carbonic acid, which is converted into carbon dioxide and water by carbonic anhydrase. Carbon dioxide diffuses out of the erythrocytes and into the plasma, where it can further diffuse across the respiratory membrane into the alveoli to be exhaled during pulmonary ventilation.
Carbaminohemoglobin
About 20 percent of carbon dioxide is bound by hemoglobin and is transported to the lungs. Carbon dioxide does not bind to iron as oxygen does; instead, carbon dioxide binds amino acid moieties on the globin portions of hemoglobin to form carbaminohemoglobin , which forms when hemoglobin and carbon dioxide bind. When hemoglobin is not transporting oxygen, it tends to have a bluish-purple tone to it, creating the darker maroon color typical of deoxygenated blood. The following formula depicts this reversible reaction:
Similar to the transport of oxygen by heme, the binding and dissociation of carbon dioxide to and from hemoglobin is dependent on the partial pressure of carbon dioxide. Because carbon dioxide is released from the lungs, blood that leaves the lungs and reaches body tissues has a lower partial pressure of carbon dioxide than is found in the tissues. As a result, carbon dioxide leaves the tissues because of its higher partial pressure, enters the blood, and then moves into red blood cells, binding to hemoglobin. In contrast, in the pulmonary capillaries, the partial pressure of carbon dioxide is high compared to within the alveoli. As a result, carbon dioxide dissociates readily from hemoglobin and diffuses across the respiratory membrane into the air.
In addition to the partial pressure of carbon dioxide, the oxygen saturation of hemoglobin and the partial pressure of oxygen in the blood also influence the affinity of hemoglobin for carbon dioxide. The Haldane effect is a phenomenon that arises from the relationship between the partial pressure of oxygen and the affinity of hemoglobin for carbon dioxide. Hemoglobin that is saturated with oxygen does not readily bind carbon dioxide. However, when oxygen is not bound to heme and the partial pressure of oxygen is low, hemoglobin readily binds to carbon dioxide.
External Website

Watch this video to see the transport of oxygen from the lungs to the tissues. Why is oxygenated blood bright red, whereas deoxygenated blood tends to be more of a purple color?
Chapter Review
Oxygen is primarily transported through the blood by erythrocytes. These cells contain a metalloprotein called hemoglobin, which is composed of four subunits with a ring-like structure. Each subunit contains one atom of iron bound to a molecule of heme. Heme binds oxygen so that each hemoglobin molecule can bind up to four oxygen molecules. When all of the heme units in the blood are bound to oxygen, hemoglobin is considered to be saturated. Hemoglobin is partially saturated when only some heme units are bound to oxygen. An oxygen–hemoglobin saturation/dissociation curve is a common way to depict the relationship of how easily oxygen binds to or dissociates from hemoglobin as a function of the partial pressure of oxygen. As the partial pressure of oxygen increases, the more readily hemoglobin binds to oxygen. At the same time, once one molecule of oxygen is bound by hemoglobin, additional oxygen molecules more readily bind to hemoglobin. Other factors such as temperature, pH, the partial pressure of carbon dioxide, and the concentration of 2,3-bisphosphoglycerate can enhance or inhibit the binding of hemoglobin and oxygen as well. Fetal hemoglobin has a different structure than adult hemoglobin, which results in fetal hemoglobin having a greater affinity for oxygen than adult hemoglobin.
Carbon dioxide is transported in blood by three different mechanisms: as dissolved carbon dioxide, as bicarbonate, or as carbaminohemoglobin. A small portion of carbon dioxide remains. The largest amount of transported carbon dioxide is as bicarbonate, formed in erythrocytes. For this conversion, carbon dioxide is combined with water with the aid of an enzyme called carbonic anhydrase. This combination forms carbonic acid, which spontaneously dissociates into bicarbonate and hydrogen ions. As bicarbonate builds up in erythrocytes, it is moved across the membrane into the plasma in exchange for chloride ions by a mechanism called the chloride shift. At the pulmonary capillaries, bicarbonate re-enters erythrocytes in exchange for chloride ions, and the reaction with carbonic anhydrase is reversed, recreating carbon dioxide and water. Carbon dioxide then diffuses out of the erythrocyte and across the respiratory membrane into the air. An intermediate amount of carbon dioxide binds directly to hemoglobin to form carbaminohemoglobin. The partial pressures of carbon dioxide and oxygen, as well as the oxygen saturation of hemoglobin, influence how readily hemoglobin binds carbon dioxide. The less saturated hemoglobin is and the lower the partial pressure of oxygen in the blood is, the more readily hemoglobin binds to carbon dioxide. This is an example of the Haldane effect.
Interactive Link Questions
When oxygen binds to the hemoglobin molecule, oxyhemoglobin is created, which has a red color to it. Hemoglobin that is not bound to oxygen tends to be more of a blue–purple color. Oxygenated blood traveling through the systemic arteries has large amounts of oxyhemoglobin. As blood passes through the tissues, much of the oxygen is released into systemic capillaries. The deoxygenated blood returning through the systemic veins, therefore, contains much smaller amounts of oxyhemoglobin. The more oxyhemoglobin that is present in the blood, the redder the fluid will be. As a result, oxygenated blood will be much redder in color than deoxygenated blood.
Review Questions
Critical thinking questions.
1. Compare and contrast adult hemoglobin and fetal hemoglobin.
2. Describe the relationship between the partial pressure of oxygen and the binding of oxygen to hemoglobin.
3. Describe three ways in which carbon dioxide can be transported.
Answers for Critical Thinking Questions
- Both adult and fetal hemoglobin transport oxygen via iron molecules. However, fetal hemoglobin has about a 20-fold greater affinity for oxygen than does adult hemoglobin. This is due to a difference in structure; fetal hemoglobin has two subunits that have a slightly different structure than the subunits of adult hemoglobin.
- The relationship between the partial pressure of oxygen and the binding of hemoglobin to oxygen is described by the oxygen–hemoglobin saturation/dissociation curve. As the partial pressure of oxygen increases, the number of oxygen molecules bound by hemoglobin increases, thereby increasing the saturation of hemoglobin.
- Carbon dioxide can be transported by three mechanisms: dissolved in plasma, as bicarbonate, or as carbaminohemoglobin. Dissolved in plasma, carbon dioxide molecules simply diffuse into the blood from the tissues. Bicarbonate is created by a chemical reaction that occurs mostly in erythrocytes, joining carbon dioxide and water by carbonic anhydrase, producing carbonic acid, which breaks down into bicarbonate and hydrogen ions. Carbaminohemoglobin is the bound form of hemoglobin and carbon dioxide.
This work, Anatomy & Physiology, is adapted from Anatomy & Physiology by OpenStax , licensed under CC BY . This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images, from Anatomy & Physiology by OpenStax , are licensed under CC BY except where otherwise noted.
Access the original for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction .
Anatomy & Physiology Copyright © 2019 by Lindsay M. Biga, Staci Bronson, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Kristen Oja, Devon Quick, Jon Runyeon, OSU OERU, and OpenStax is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License , except where otherwise noted.
- MSD careers
Exchanging Oxygen and Carbon Dioxide
The primary function of the respiratory system is to take in oxygen and eliminate carbon dioxide. Inhaled oxygen enters the lungs and reaches the alveoli. The layers of cells lining the alveoli and the surrounding capillaries are each only one cell thick and are in very close contact with each other. This barrier between air and blood averages about 1 micron ( 1 / 10,000 of a centimeter, or 0.000039 inch) in thickness. Oxygen passes quickly through this air-blood barrier into the blood in the capillaries. Similarly, carbon dioxide passes from the blood into the alveoli and is then exhaled.
Oxygenated blood travels from the lungs through the pulmonary veins and into the left side of the heart, which pumps the blood to the rest of the body (see Function of the Heart ). Oxygen-deficient, carbon dioxide-rich blood returns to the right side of the heart through two large veins, the superior vena cava and the inferior vena cava. Then the blood is pumped through the pulmonary artery to the lungs, where it picks up oxygen and releases carbon dioxide.
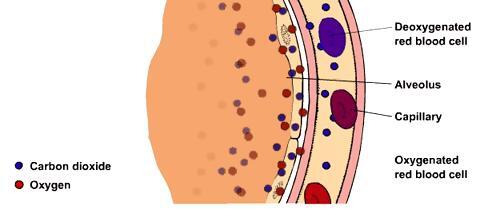
To support the absorption of oxygen and release of carbon dioxide, about 5 to 8 liters (about 1.3 to 2.1 gallons) of air per minute are brought in and out of the lungs, and about three tenths of a liter (about three tenths of a quart) of oxygen is transferred from the alveoli to the blood each minute, even when the person is at rest. At the same time, a similar volume of carbon dioxide moves from the blood to the alveoli and is exhaled. During exercise, it is possible to breathe in and out more than 100 liters (about 26 gallons) of air per minute and extract 3 liters (a little less than 1 gallon) of oxygen from this air per minute. The rate at which oxygen is used by the body is one measure of the rate of energy expended by the body. Breathing in and out is accomplished by respiratory muscles .
Gas Exchange Between Alveolar Spaces and Capillaries
Three processes are essential for the transfer of oxygen from the outside air to the blood flowing through the lungs: ventilation, diffusion, and perfusion.
Ventilation is the process by which air moves in and out of the lungs.
Diffusion is the spontaneous movement of gases, without the use of any energy or effort by the body, between the alveoli and the capillaries in the lungs.
Perfusion is the process by which the cardiovascular system pumps blood throughout the lungs.
The body's circulation is an essential link between the atmosphere, which contains oxygen, and the cells of the body, which consume oxygen. For example, the delivery of oxygen to the muscle cells throughout the body depends not only on the lungs but also on the ability of the blood to carry oxygen and on the ability of the circulation to transport blood to muscle. In addition, a small fraction of the blood pumped from the heart enters the bronchial arteries and nourishes the airways.
Heart–Lung Connections

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Cookie Preferences
Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science ⋅
- Biology ⋅
How Do Humans Get Oxygen in Their Bodies?

Why is Breathing Important to Organisms?
Nearly every organism on the planet needs oxygen. Some get it through water and others, like humans, get it through breathing air. Human energy comes from food and oxygen, but food only gives us 10 percent of our energy needs. Oxygen is required for other 90 percent or our energy, and every cell in the body requires oxygen to live. For the body to receive oxygen, the respitory system, heart, cells, and arteries and veins must play an active role.
Respiratory System
The respiratory system is the gateway that allows oxygen to enter your body. The mouth, nose, trachea, lungs and diaphragm all participate in oxygen absorption. Oxygen enters the body in the mouth and nose, passes through the larynx and the trachea. The trachea splits into two bronchial tubes, which lead to smaller tubes that lead to 600 million alveoli, which are small sacs surrounded by capillaries. The capillaries take oxygen into the arteries, and the oxygen-rich blood is then pumped into every cell of your body. Once the oxygen has been absorbed, carbon dioxide and water are eliminated through the lungs.
The cells are oxidized through an enzymatic processes, and the oxidation is the source of energy for humans and most other mammals. Oxygen is needed to build new cells and tissue, replace old tissue, dispose of waste material and reproduce more cells.
The heart is the powerhouse that sends oxygen pumping through your body to each and every cell. Before each heartbeat, the heart fills with blood. The muscle then contracts to expel the blood into arteries. The left side of the heart sends oxygen-rich blood to the body, and the right side sends the depleted blood, filled with carbon dioxide, to the lungs to be expelled. Your heart beats consistently, for your entire life, never allowing the oxygen to deplete.
Arteries and Veins
The arteries are the passageways that take the five liters of rich oxygenated blood away from the heart, throughout the body. The vessels that take the blood back to the heart are called veins. It takes about 60 seconds for the heart to pump oxygen-filled blood through the entire body.
Related Articles
How does blood get oxygen, the respiratory & circulatory system in the human body, what do our body cells do with oxygen, functions of human organs, how do the respiratory & cardiovascular system work..., what are the functions of alveoli in the lungs, relationship between calories & cellular respiration, what is the purpose of breathing, how does an octopus breathe, how are photosynthesis & cellular respiration related, which organs help the human body get rid of wastes..., which part of the body makes blood, how is oxygen important to the release of energy in..., why is chemistry important to the study of anatomy..., the disadvantages of lactic acid fermentation, structure of the cardiovascular system, where does respiration occur, the highest partial pressure of oxygen in the circulatory..., body parts & functions.
- Kids Health: Your Hear and Circulatory System
- Oxygen Review: Human Body
- The Franklin Institute: Respiratory System: Oxygen Delivery System
- The Franklin Institute: Circulatory System: The Circle of Blood
- The Franklin Institute: Coronary Circulation: It's All in the Heart
- The Franklin Institute: Systemic Circulation: It's All Throughout the Body
About the Author
Veronica Maier has been an active online writer since 2010. She has been a contributing writer to eHow and Answerbag. Maier holds a Bachelor of Arts in art history and visual culture with an emphasis on the American modern from the University of California, Santa Cruz.
Photo Credits
Dynamic Graphics/Creatas/Getty Images
Find Your Next Great Science Fair Project! GO
Advertisement
How Your Lungs Work
- Share Content on Facebook
- Share Content on LinkedIn
- Share Content on Flipboard
- Share Content on Reddit
- Share Content via Email
Where the Air Goes
As you breathe air in through your nose or mouth, it goes past the epiglottis and into the trachea . It continues down the trachea through your vocal cords in the larynx until it reaches the bronchi . From the bronchi, air passes into each lung. The air then follows narrower and narrower bronchioles until it reaches the alveoli .
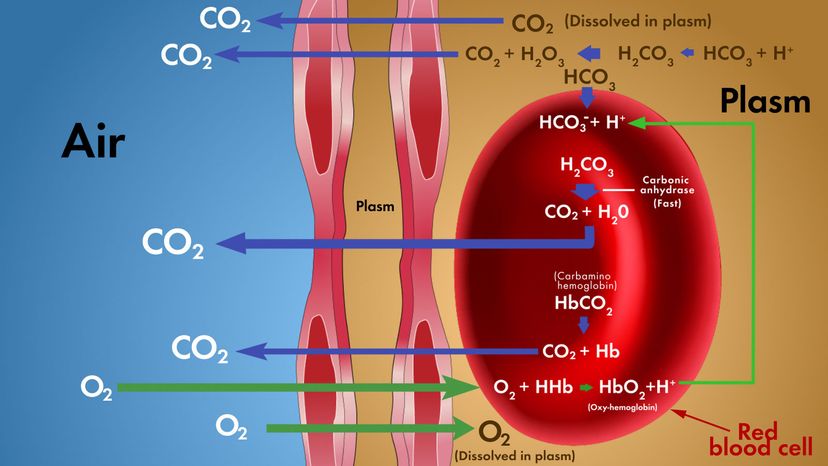
Within each air sac, the oxygen concentration is high, so oxygen passes or diffuses across the alveolar membrane into the pulmonary capillary . At the beginning of the pulmonary capillary, the hemoglobin in the red blood cells has carbon dioxide bound to it and very little oxygen (see illustration above). The oxygen binds to hemoglobin and the carbon dioxide is released. Carbon dioxide is also released from sodium bicarbonate dissolved in the blood of the pulmonary capillary. The concentration of carbon dioxide is high in the pulmonary capillary, so carbon dioxide leaves the blood and passes across the alveolar membrane into the air sac. This exchange of gases occurs rapidly (fractions of a second). The carbon dioxide then leaves the alveolus when you exhale and the oxygen-enriched blood returns to the heart. Thus, the purpose of breathing is to keep the oxygen concentration high and the carbon dioxide concentration low in the alveoli so this gas exchange can occur!
Anatomy of the Lung
- alveolus - tiny, thin-walled air sac at the end of the bronchiole branches where gas exchange occurs (plural - alveoli).
- bronchioles - numerous small tubes that branch from each bronchus into the lungs. They get smaller and smaller.
- bronchus - a branch of the trachea that goes from the trachea into the lung (plural - bronchi)
- diaphragm - muscle at the base of the chest cavity that contracts and relaxes during breathing
- epiglottis - a flap of tissue that closes over the trachea when you swallow so that food does not enter your airway
- intercostal muscles - muscles along the rib cage that assist in breathing
- larynx - voice box where the vocal cords are located.
- nasal cavity - chamber in from the nose where air is moistened and warmed
- pleural membranes - thin, membranes that cover the lungs, separate them from other organs and form a fluid-filled chest cavity.
- pulmonary capillaries - small blood vessels that surround each alveolus
- trachea -rigid tube that connects the mouth with the bronchi (windpipe)
Please copy/paste the following text to properly cite this HowStuffWorks.com article:

IMAGES
VIDEO
COMMENTS
Learn how oxygen travels from the air to your cells through your gut, brain, bones, lungs, blood, and heart. Watch a TEDEd video that explains the complex journey of oxygen in your body.
Oxygen is mainly transported by hemoglobin, a protein in red blood cells, which binds and releases oxygen depending on the partial pressure of oxygen in the lungs. The oxygen-dissociation curve shows how hemoglobin's affinity for oxygen changes with various factors, such as pH, carbon dioxide, and temperature.
View full lesson: http://ed.ted.com/lessons/oxygen-s-surprisingly-complex-journey-through-your-body-enda-butlerOxygen forms about 21% of the air around us. I...
Oxygen is essential for adenosine triphosphate (ATP) generation through oxidative phosphorylation; therefore, it must be reliably delivered to all metabolically active cells in the body.[1][2] In the setting of hypoxia or low blood oxygen levels, irreversible tissue damage can rapidly occur. Hypoxia can result from an impaired oxygen-carrying capacity of the blood (eg, anemia), impaired ...
Presenter Zoe Gamble explains how oxygen is transported around the body in our blood.Graphics show how when we exercise, our lungs work harder to take in oxy...
Chapter 2. The Circulatory System and Oxygen Transport. The cardiovascular or circulatory system is designed to ensure the survival of all cells of the body at every moment and it does this by maintaining the immediate chemical environment of each cell in the body (i.e., the interstitial fluid) at a composition appropriate for that cell's ...
Oxygen forms about 21% of the air around us. In your body, oxygen forms a vital role in the production of energy in most cells. But if gases can only efficiently diffuse across tiny distances, how does oxygen reach the cells deep inside your body? Enda Butler tracks the surprisingly complex journey of oxygen through your body.
Learn how oxygen is transported in blood by hemoglobin, and how partial pressure, pH, and temperature affect the binding and release of oxygen. The web page does not provide the direct answer to the query, but explains the factors that influence the oxygen-hemoglobin dissociation curve.
Learn how the respiratory system provides oxygen to the cells for aerobic metabolism and removes carbon dioxide from the body. Explore the structure and function of the lungs, blood, and mitochondria in gas exchange and energy production.
Learn how haemoglobin, a tetrameric protein, binds oxygen and delivers it to tissues in vertebrates. Explore the factors that affect haemoglobin-oxygen affinity, such as pH, temperature and 2,3-DPG, and the clinical implications of carbon monoxide poisoning.
Learn how the heart, lungs, and circulation extract oxygen from the atmosphere and deliver it to the tissues to maintain aerobic metabolism. The article covers the key steps in oxygen transport, the carrying capacity of blood, the regional distribution of oxygen, and the cellular use of oxygen.
Learn how air travels into your body through your nose or mouth, past your voice box and windpipe, and into your lungs. See how gas exchange takes place at the alveoli, where oxygen enters your blood and carbon dioxide leaves your body.
The oxygen in inhaled air passes across the thin lining of the air sacs and into the blood vessels. This is known as diffusion. The oxygen in the blood is then carried around the body in the bloodstream, reaching every cell. When oxygen passes into the bloodstream from the air sacs, carbon dioxide leaves the blood and passes into the air sacs.
Learn how your lungs perform gas exchange, temperature regulation, protection, and smell. Oxygen is essential for every cell in your body and your lungs deliver it through your bloodstream.
Learn how oxygen and carbon dioxide are transferred between the alveoli and the blood in the lungs, and how the respiratory and cardiovascular systems support this process. Find out how ventilation, diffusion, and perfusion work together, and how they are affected by lung and airway disorders.
A small amount of oxygen does dissolve in the blood and is transported in the bloodstream, but it is only about 1.5% of the total amount. The majority of oxygen molecules are carried from the lungs to the body's tissues by a specialized transport system, which relies on the erythrocyte—the red blood cell.
Learn how the respiratory system enables oxygen to enter the body and carbon dioxide to leave the body. See a 3D model of the respiratory system and its parts, such as the nose, mouth, throat, trachea, bronchi, lungs, and alveoli.
Learn how oxygen is transported from the lungs to the body's tissues by hemoglobin, a protein in red blood cells. Find out how partial pressure, pH, and temperature affect the binding and dissociation of oxygen to hemoglobin.
The oxygen cascade describes the physiological steps that bring atmospheric oxygen into the body where it is delivered and consumed by metabolically active tissue. The review highlights how the cascade adapts to exercise, altitude, and disease, and how it affects oxygen consumption and breathing.
Oxygenated blood travels from the lungs through the pulmonary veins and into the left side of the heart, which pumps the blood to the rest of the body (see Function of the Heart). Oxygen-deficient, carbon dioxide-rich blood returns to the right side of the heart through two large veins, the superior vena cava and the inferior vena cava.
Oxygen enters the body through the mouth and nose, and passes through the respiratory system, heart, cells, and arteries and veins. Learn how oxygen is absorbed, transported, and used by the body for energy and cell function.
Learn how air flows through your nose, mouth, trachea, bronchi, bronchioles and alveoli, and how oxygen and carbon dioxide are exchanged in your lungs. See illustrations and definitions of the respiratory system anatomy.